When Other Treatments Have Failed, There Is Hope
Columbia Associates is proud to partner with NeuroStar® to provide transcranial magnetic stimulation (TMS) as a treatment option for our clients. It is FDA-cleared to treat depression, depression with anxiety, and OCD. Most major insurance plans cover NeuroStar® TMS, including Medicare and Tricare plans nationwide.
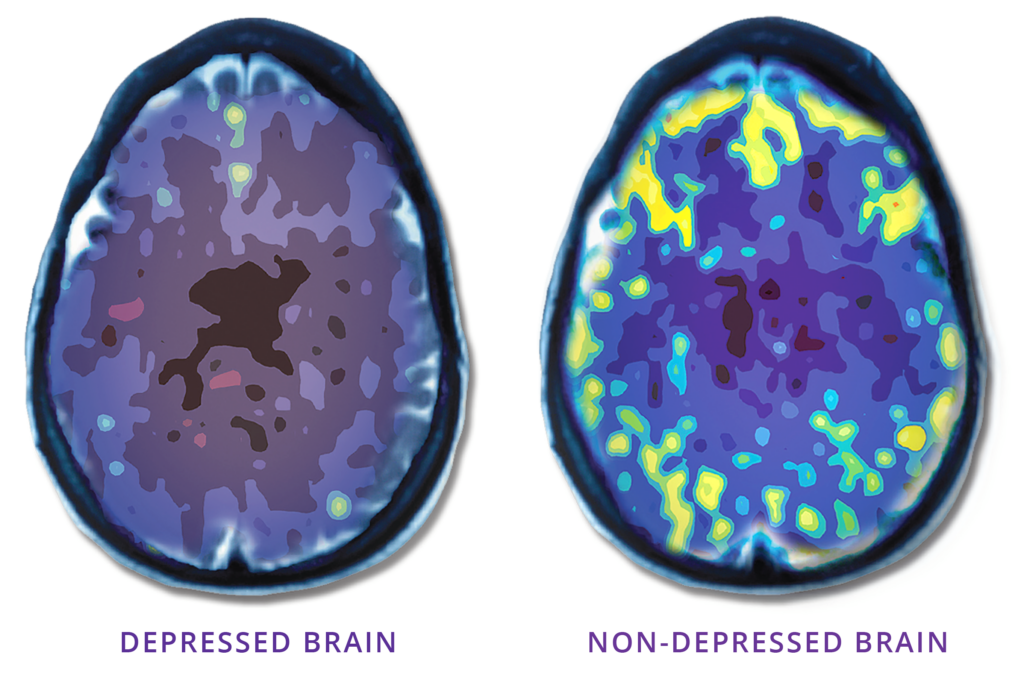
TMS uses focused magnetic pulses, similar in strength to an MRI, to revitalize underactive areas of the brain involved in regulating mood. It is not electroconvulsive therapy (ECT), “shock therapy,” or surgery.
How NeuroStar® TMS Can Help
NeuroStar® TMS has helped thousands of people with depression get relief from their depression symptoms.
6.9
million NeuroStar® Advanced TMS treatments have been performed in over 188,000 patients
83%
of patients experience an improvement in the severity of their depression
62%
of patients show complete remission
Neurostar® TMS can offer hope to those who have struggled to find relief from their depression symptoms.
If you have been diagnosed with major depressive disorder (MDD) and have not experienced relief from antidepressants, NeuroStar® TMS might be right for you. NeuroStar® TMS treatment is also covered by insurance for more than 300 million people.
How NeuroStar® TMS Therapy Works
TMS treatment is conducted in one of our Columbia Associates clinics. You will meet with our team for a consultation to determine your eligibility. Depending on your provider’s recommendation, a typical TMS treatment plan includes 36 sessions that each last between 19–37 minutes. Completing the full treatment course gives the best chance for delivering long-lasting relief from depression.
- Before treatment – You’ll recline comfortably in the treatment chair. A curved magnetic coil will be positioned on your head.
- During treatment – You’ll hear a clicking sound and feel a tapping sensation on your head as the NeuroStar® coil focuses treatment precisely at the source of the depression: the brain. The patented Contact Sensing technology ensures the prescribed dose is delivered, every treatment, every pulse.
- After treatment – With NeuroStar® TMS, you are fully awake and alert during treatment and may drive home and resume daily activities immediately afterward. There are no negative effects on memory or sleep.
With Columbia Associates, you’re supported every step of the way on your journey to regain control and live a fulfilling life free from the constraints of depression.
Find out if NeuroStar® TMS is right for you.
Frequently Asked Questions
TMS Therapy for Adolescent Depression Treatment
Neurostar® TMS is a promising option, available to adolescents aged 15–21 as an add-on therapy for treatment-resistant depression (TRD). We understand that managing depression can be incredibly challenging for young people, and our team is dedicated to supporting families through every step of the treatment process. NeuroStar® provides a non-invasive and non-systemic approach to targeting the areas of the brain that affect mood regulation. TMS therapy significantly alleviates depressive symptoms without the side effects commonly associated with antidepressant medications.
At Columbia Associates, our caring professionals are trained to deliver this advanced therapy, ensuring a supportive and nurturing environment for adolescents undergoing TMS therapy. Call us at 703.682.8208 or complete the TMS form to learn more about our treatment options.
Find out if NeuroStar® TMS is right for you.
Discover How NeuroStar® TMS Can Improve Your Life
At Columbia Associates, we’re here to help you find the proper depression treatment. We are proud to offer a full suite of mental health services, including therapy and psychiatric medication management, as well as alternative treatment options for depression like NeuroStar® TMS and Spravato®. This collaborative, multidisciplinary approach allows our team to create an individualized treatment plan that is tailored to you.
If you’re ready to take the next step in your mental health journey, consider NeuroStar® TMS. With TMS, you may finally discover the relief you’ve sought. Call us at 703.682.8208 or complete the TMS form.
Indication Statement
The NeuroStar® Advanced Therapy system is indicated for the treatment of depressive episodes and for decreasing anxiety symptoms for those who may exhibit comorbid anxiety symptoms in adult patients suffering from major depressive disorder and who failed to achieve satisfactory improvement from previous antidepressant medication treatment in the current episode.
The NeuroStar® Advanced Therapy system is intended to be used as an adjunct for the treatment of adult patients suffering from obsessive-compulsive disorder.
NeuroStar® Advanced Therapy is only available by prescription. A doctor can help decide if NeuroStar® Advanced Therapy is right for you. Patients’ results may vary.
The most common side effect is pain or discomfort at or near the treatment site. These events are transient; they occur during the TMS treatment course and do not occur for most patients after the first week of treatment. There is a rare risk of seizure associated with the use of TMS therapy (<0.1% per patient).
Visit NeuroStar.com for full safety and prescribing information.
Clinical and Academic References
- Carpenter LL, et al. (2012). Transcranial Magnetic Stimulation (TMS) for Major Depression: A Multisite, Naturalistic, Observational Study of Acute Treatment Outcomes in Clinical Practice. Depression and Anxiety, 29(7):587-596. www.ncbi.nlm.nih.gov/pubmed/22689344
- George MS, et al. (2010). Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial. Arch Gen Psychiatry, 67(5):507-516. www.ncbi.nlm.nih.gov/pubmed/20439832
- Dunner DL, et al. (2014). A Multisite, Naturalistic, Observational Study of Transcranial Magnetic Stimulation (TMS) for Patients with Pharmacoresistant Major Depressive Disorder: Durability of Benefit Over a 1-Year Follow-Up Period. J Clin Psychiatry, 75(12):1394-1401. www.ncbi.nlm.nih.gov/pubmed/25271871
- O’Reardon JP, et al. (2007). Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol Psychiatry, 62(11):1208-1216. www.ncbi.nlm.nih.gov/pubmed/17573044

